sodium number of protons|How to find the Number of Protons, Electrons, Neutrons for : Pilipinas Mar 8, 2020 — In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sodium (Na). From the Periodic.
SpankBang is a popular adult video streaming site that you can watch with VLC media player. To do that, you need to install the SpankBang URL parser, a simple Lua script that you can download from this page. Follow the instructions to place it in the right directory and enjoy your videos.
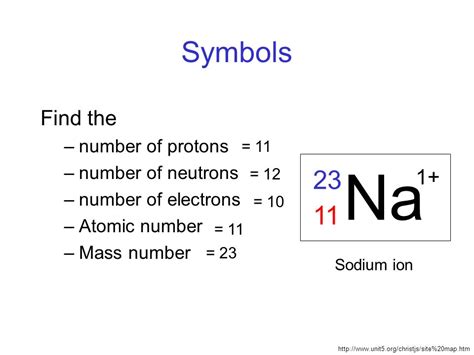
sodium number of protons,Dis 8, 2020 — Learn about the number of protons, neutrons, and electrons in sodium, as well as its electron configuration and oxidation states. Find out the main isotopes of sodium, their half-lives, decay modes, and applications.Ago 22, 2024 — Sodium is the 11th element of the periodic table so its atomic number is 11. Therefore, a sodium atom has eleven protons, twelve neutrons and eleven electrons.Sodium is the 11th element in the periodic table and has a symbol of Na and atomic number of 11. It has eleven protons and twelve neutrons in its nucleus, and eleven electrons in three shells.

Crystal Structure: Cubic. Density @ 293 K: 0.971 g/cm 3. Color: silvery. Atomic Structure. Isotopes. Facts. Date of Discovery: 1807. Discoverer: Sir Humphrey Davy. Name Origin: soda .
sodium number of protons How to find the Number of Protons, Electrons, Neutrons for Crystal Structure: Cubic. Density @ 293 K: 0.971 g/cm 3. Color: silvery. Atomic Structure. Isotopes. Facts. Date of Discovery: 1807. Discoverer: Sir Humphrey Davy. Name Origin: soda .
Element Sodium (Na), Group 1, Atomic Number 11, s-block, Mass 22.990. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
Mar 8, 2020 — In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sodium (Na). From the Periodic.Nob 21, 2020 — Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na.
How to find the Number of Protons, Electrons, Neutrons for Characteristics. Physical. Emission spectrum for sodium, showing the D line. Sodium at standard temperature and pressure is a soft silvery metal that combines with oxygen in the air, forming sodium oxides. Bulk sodium is .Sodium is a chemical element with symbol Na and atomic number 11. It is a soft, silver-white, highly reactive metal and belongs to group 1 (alkali metals) in the periodic table.
The chemical abbreviation for sodium was first published by Jöns Jakob Berzelius in his system of atomic symbols. It is a contraction of the element's new Latin name natrium, which refers to the Egyptian natron, a natural mineral salt .
Mar 8, 2020 — In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sodium (Na). From the Periodic.We know that the mass number (A) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: p = 35 – 18 = 17, and therefore, the element is Cl The Number of Protons from Electrons. For a .This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element. The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons.The number of protons in the nucleus of an atom is its atomic number (\(Z\)). This is the defining trait of an element: Its value determines the identity of the atom. . For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom .The mass number close mass number The number of protons and neutrons found in the nucleus of an atom. is given at the top left of the elements symbol, for example, sodium has a mass number of 23.
Mar 20, 2023 — The atomic number is the number of protons and electrons equal to protons located outside the nucleus. That is, we can finally say that there are electrons equal to the atomic number in the sodium atom. From the periodic table, we see that the atomic number of sodium is 11. That is, a sodium atom has a total of eleven electrons.Number of Protons: 11: Number of Neutrons: 12: Number of Electrons: 11: Melting Point: 97.88° C: Boiling Point: 552.9° C: Density: 2.62 grams per cubic centimeter: Normal Phase: Solid: . Sodium hydroxide or caustic soda (NaOH) Sodium nitrate or Chilean saltpeter (NaNO 3) Sodium Borate or borax (Na 2 B 4 O 7) Interesting facts:Set 22, 2023 — This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element. . For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons. If you change the atomic number to 12, you are no longer dealing with sodium atoms, but magnesium atoms. .Hun 26, 2024 — The number of protons gives an atom its identity. Protons are positively charged particles that are found in the nucleus of an atom. No two elements have the same number of protons. An atom with 1 proton is a hydrogen atom; an atom with 11 .Nob 21, 2020 — Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na . The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons .
Ago 26, 2020 — Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass .Each element has a unique number of protons. An element's atomic number is equal to the number of protons in the nuclei of any of its atoms. The mass number of an atom is the sum of the protons and neutrons in the atom. Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons in their atomic nuclei.sodium number of protonsEach isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass unit ( amu ), .

Hul 21, 2022 — Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons must equal the number of protons in a neutral atom). Subtracting 11 (the number of electrons) from 11 (the number of protons) gives zero, the overall charge on the neutral atom. See the right side of Figure \(\PageIndex{1 .Number of protons in a sodium nucleus = 11 (the atomic number)Number of electrons orbiting a sodium nucleus = 11. (the atom is neutral)Number of neutrons in a sodium nucleus = 23 – 11 = 12.
May 28, 2024 — The number of protons present in an atom is defined by the element's atomic number. Therefore, every atom of tungsten contains 74 protons. . When using this notation, the symbol of the element must be used to find its atomic number. Since sodium (Na) has an atomic number of 11, .Dis 13, 2023 — Determine the number of protons and electrons in an atom. Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. . For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom (Z = 8 .Mar 23, 2023 — Protons, neutrons and electrons of all elements are mentioned in the table below (You will get the List + Shell diagram of all the elements.) . Sodium has 11 protons, 12 neutrons and 11 electrons: 12: Magnesium has 12 protons, 12 neutrons and 12 electrons: 13:
sodium number of protons|How to find the Number of Protons, Electrons, Neutrons for
PH0 · Sodium – Atomic Number – Na
PH1 · Sodium (Na)
PH2 · Sodium
PH3 · How to find the Number of Protons, Electrons, Neutrons for
PH4 · How Many Protons, Neutrons and Electrons Does
PH5 · Chemical Elements.com